3d drawing of hydrogen fulminate
1:2 Electron-Dot Model of Bonding: Lewis Structures and Formal Charges
- Page ID
- 89969
Using Lewis Dot Symbols to Describe Covalent Bonding 
This sharing of electrons allowing atoms to "stick" together is the basis of covalent bonding. In that location is some intermediate distant, mostly a fleck longer than 0.1 nm, or if you prefer 100 pm, at which the attractive forces significantly outweigh the repulsive forces and a bond will be formed if both atoms can achieve a completen s two np 6 configuration. It is this beliefs that Lewis captured in his octet rule. The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its construction, stoichiometry, and backdrop. For example, chlorine, with seven valence electrons, is i electron short of an octet. If ii chlorine atoms share their unpaired electrons by making a covalent bond and forming Cltwo, they tin can each consummate their valence shell:
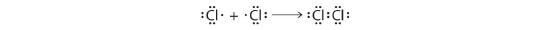
Each chlorine cantlet at present has an octet. The electron pair existence shared by the atoms is chosen a bonding pair ; the other three pairs of electrons on each chlorine atom are called lone pairs. Lone pairs are not involved in covalent bonding. If both electrons in a covalent bond come from the same atom, the bail is called a coordinate covalent bail.
We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols:
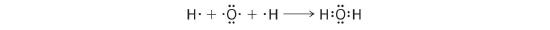
The structure on the right is the Lewis electron structure, or Lewis structure, for H2O. With two bonding pairs and two alone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence vanquish of two electrons. Chemists commonly point a bonding pair by a single line, as shown hither for our ii examples:

The following procedure can be used to construct Lewis electron structures for more complex molecules and ions:
- Adjust the atoms to evidence specific connections. When at that place is a central cantlet, information technology is usually the to the lowest degree electronegative element in the chemical compound. Chemists commonly list this key atom beginning in the chemical formula (as in CCl4 and COiii ii−, which both have C every bit the central cantlet), which is another clue to the compound'due south construction. Hydrogen and the halogens are most always connected to just ane other atom, and so they are normally terminal rather than fundamental.
Notation
The central atom is usually the least electronegative element in the molecule or ion; hydrogen and the halogens are ordinarily terminal.
- Make up one's mind the full number of valence electrons in the molecule or ion. Add the valence electrons from each atom. (Recall from Chapter ii that the number of valence electrons is indicated past the position of the element in the periodic table.) If the species is a polyatomic ion, call up to add or subtract the number of electrons necessary to requite the total charge on the ion. For CO32−, for example, nosotros add two electrons to the total considering of the −2 accuse.
- Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. In \(H_2O\), for case, there is a bonding pair of electrons betwixt oxygen and each hydrogen.
- First with the terminal atoms, add enough electrons to each cantlet to requite each atom an octet (two for hydrogen). These electrons will usually exist solitary pairs.
- If whatever electrons are left over, identify them on the fundamental atom. Some atoms are able to accommodate more than eight electrons.
- If the central atom has fewer electrons than an octet, use lone pairs from concluding atoms to form multiple (double or triple) bonds to the fundamental atom to achieve an octet. This volition not change the number of electrons on the terminal atoms.
Now let's apply this procedure to some particular compounds, beginning with one nosotros take already discussed.
H2O 
1. Because H atoms are nigh always last, the organisation within the molecule must exist HOH.
2. Each H cantlet (group 1) has 1 valence electron, and the O atom (group sixteen) has half-dozen valence electrons, for a total of 8 valence electrons.
3. Placing one bonding pair of electrons between the O cantlet and each H atom gives H:O:H, with 4 electrons left over.
4. Each H atom has a full valence crush of 2 electrons.
5. Adding the remaining four electrons to the oxygen (as two lone pairs) gives the post-obit construction:
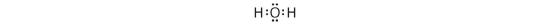
This is the Lewis structure we drew earlier. Considering it gives oxygen an octet and each hydrogen two electrons, we practise not need to use pace vi.
OCl− 
1. With only 2 atoms in the molecule, there is no central atom.
2. Oxygen (group 16) has 6 valence electrons, and chlorine (group 17) has 7 valence electrons; we must add one more than for the negative charge on the ion, giving a total of 14 valence electrons.
3. Placing a bonding pair of electrons betwixt O and Cl gives O:Cl, with 12 electrons left over.
4. If we place half-dozen electrons (as three lonely pairs) on each atom, we obtain the post-obit structure:
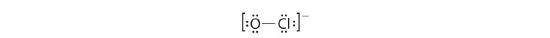
Each atom now has an octet of electrons, so steps v and six are not needed. The Lewis electron construction is fatigued within brackets as is customary for an ion, with the overall charge indicated outside the brackets, and the bonding pair of electrons is indicated past a solid line. OCl− is the hypochlorite ion, the active ingredient in chlorine laundry bleach and swimming pool disinfectant.
CH2O 
1. Considering carbon is less electronegative than oxygen and hydrogen is normally concluding, C must exist the central atom. One possible arrangement is as follows:
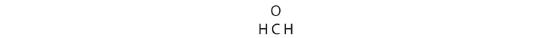
two. Each hydrogen atom (group 1) has one valence electron, carbon (grouping xiv) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [(2)(ane) + 4 + 6] = 12 valence electrons.
3. Placing a bonding pair of electrons between each pair of bonded atoms gives the post-obit:
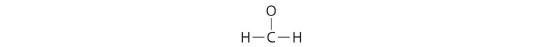
Six electrons are used, and 6 are left over.
4. Calculation all 6 remaining electrons to oxygen (as three alone pairs) gives the following:

Although oxygen at present has an octet and each hydrogen has 2 electrons, carbon has only half dozen electrons.
5. There are no electrons left to identify on the key cantlet.
6. To give carbon an octet of electrons, nosotros use one of the alone pairs of electrons on oxygen to grade a carbon–oxygen double bond:

Both the oxygen and the carbon now take an octet of electrons, so this is an adequate Lewis electron structure. The O has two bonding pairs and two lone pairs, and C has four bonding pairs. This is the structure of formaldehyde, which is used in embalming fluid.
An alternative structure can be fatigued with one H bonded to O. Formal charges, discussed later in this section, suggest that such a structure is less stable than that shown previously.
Example
Write the Lewis electron structure for each species.
- NCl3
- Sii ii−
- NOCl
Given: chemical species
Asked for: Lewis electron structures
Strategy:
Use the half-dozen-step procedure to write the Lewis electron structure for each species.
Solution:
Nitrogen is less electronegative than chlorine, and element of group vii atoms are unremarkably terminal, so nitrogen is the central cantlet. The nitrogen cantlet (group 15) has 5 valence electrons and each chlorine atom (grouping 17) has 7 valence electrons, for a total of 26 valence electrons. Using ii electrons for each North–Cl bond and adding three lone pairs to each Cl business relationship for (3 × 2) + (iii × 2 × 3) = 24 electrons. Rule 5 leads us to place the remaining ii electrons on the central N:
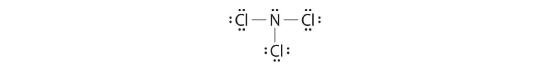
Nitrogen trichloride is an unstable oily liquid once used to bleach flour; this utilise is now prohibited in the Usa.
-
-
In a diatomic molecule or ion, we do non need to worry well-nigh a central atom. Each sulfur atom (grouping 16) contains 6 valence electrons, and we need to add 2 electrons for the −two charge, giving a total of 14 valence electrons. Using ii electrons for the S–S bond, we suit the remaining 12 electrons equally three lone pairs on each sulfur, giving each Southward atom an octet of electrons:
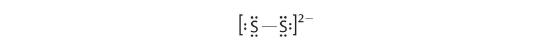
-
Because nitrogen is less electronegative than oxygen or chlorine, it is the central atom. The N atom (grouping 15) has v valence electrons, the O atom (group 16) has 6 valence electrons, and the Cl atom (group 17) has 7 valence electrons, giving a total of 18 valence electrons. Placing one bonding pair of electrons between each pair of bonded atoms uses 4 electrons and gives the post-obit:
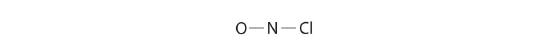
Adding three lone pairs each to oxygen and to chlorine uses 12 more electrons, leaving 2 electrons to place as a alone pair on nitrogen:
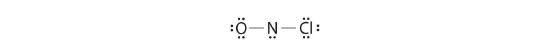
-
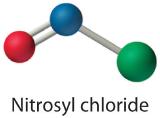
Because this Lewis structure has only 6 electrons around the central nitrogen, a lone pair of electrons on a terminal atom must be used to course a bonding pair. We could apply a solitary pair on either O or Cl. Because we accept seen many structures in which O forms a double bond but none with a double bond to Cl, information technology is reasonable to select a solitary pair from O to give the following:
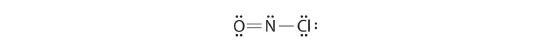
All atoms now have octet configurations. This is the Lewis electron structure of nitrosyl chloride, a highly corrosive, ruby-orange gas.
Practise
Write Lewis electron structures for COii and SCl2, a vile-smelling, unstable red liquid that is used in the manufacture of rubber.
Answer:
Formal Charges 
It is sometimes possible to write more than one Lewis construction for a substance that does not violate the octet rule, as we saw for CHiiO, but non every Lewis structure may exist equally reasonable. In these situations, we can choose the most stable Lewis structure by because the formal charge on the atoms, which is the difference between the number of valence electrons in the free atom and the number assigned to it in the Lewis electron structure. The formal charge is a way of calculating the charge distribution inside a Lewis structure; the sum of the formal charges on the atoms within a molecule or an ion must equal the overall charge on the molecule or ion. A formal charge does not represent a truthful accuse on an atom in a covalent bond but is simply used to predict the most likely structure when a compound has more than i valid Lewis construction.
To calculate formal charges, we assign electrons in the molecule to individual atoms co-ordinate to these rules:
- Nonbonding electrons are assigned to the atom on which they are located.
- Bonding electrons are divided every bit betwixt the bonded atoms.
For each atom, we and so compute a formal charge:
To illustrate this method, let's summate the formal accuse on the atoms in ammonia (NHiii) whose Lewis electron construction is equally follows:
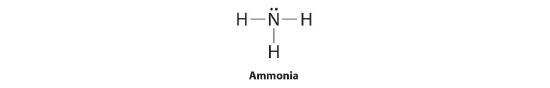
A neutral nitrogen atom has five valence electrons (it is in group fifteen). From its Lewis electron structure, the nitrogen atom in ammonia has one lonely pair and shares three bonding pairs with hydrogen atoms, so nitrogen itself is assigned a total of five electrons [two nonbonding e− + (half dozen bonding eastward−/2)]. Substituting into Equation 5.iii.1, we obtain
A neutral hydrogen atom has 1 valence electron. Each hydrogen atom in the molecule shares one pair of bonding electrons and is therefore assigned one electron [0 nonbonding e− + (two bonding east−/two)]. Using Equation iv.four.i to calculate the formal charge on hydrogen, we obtain
The hydrogen atoms in ammonia have the aforementioned number of electrons as neutral hydrogen atoms, so their formal accuse is also zip. Calculation together the formal charges should give us the overall charge on the molecule or ion. In this case, the nitrogen and each hydrogen has a formal charge of goose egg. When summed the overall charge is zero, which is consistent with the overall charge on the NH3 molecule.
Typically, the structure with the most charges on the atoms closest to zero is the more stable Lewis structure. In cases where at that place are positive or negative formal charges on various atoms, stable structures by and large have negative formal charges on the more than electronegative atoms and positive formal charges on the less electronegative atoms. The side by side example further demonstrates how to calculate formal charges.
Case
Summate the formal charges on each atom in the NHfour + ion.
Given: chemical species
Asked for: formal charges
Strategy:
Identify the number of valence electrons in each atom in the NHfour + ion. Use the Lewis electron construction of NH4 + to identify the number of bonding and nonbonding electrons associated with each cantlet then use Equation four.4.one to calculate the formal charge on each atom.
Solution:
The Lewis electron structure for the NH4 + ion is as follows:

The nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. Using Equation 4.4.1, the formal accuse on the nitrogen atom is therefore
Each hydrogen atom in has one bonding pair. The formal charge on each hydrogen cantlet is therefore
The formal charges on the atoms in the NHfour + ion are thus
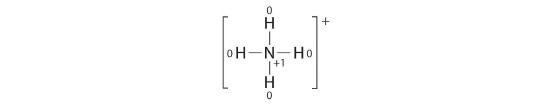
Adding together the formal charges on the atoms should give united states the full charge on the molecule or ion. In this case, the sum of the formal charges is 0 + 1 + 0 + 0 + 0 = +one.
Exercise
Write the formal charges on all atoms in BH4 −.
Reply:
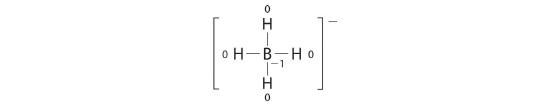
If an atom in a molecule or ion has the number of bonds that is typical for that atom (e.g., four bonds for carbon), its formal charge is nada.
Using Formal Charges to Distinguish between Lewis Structures
As an example of how formal charges can be used to determine the virtually stable Lewis structure for a substance, we tin can compare two possible structures for CO2. Both structures suit to the rules for Lewis electron structures.
COii
1. C is less electronegative than O, and so it is the central cantlet.
2. C has 4 valence electrons and each O has 6 valence electrons, for a total of sixteen valence electrons.
3. Placing one electron pair between the C and each O gives O–C–O, with 12 electrons left over.
4. Dividing the remaining electrons between the O atoms gives iii lone pairs on each atom:
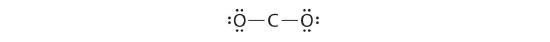
This structure has an octet of electrons effectually each O cantlet but only 4 electrons effectually the C atom.
five. No electrons are left for the primal atom.
half-dozen. To give the carbon atom an octet of electrons, we can convert two of the alone pairs on the oxygen atoms to bonding electron pairs. In that location are, nevertheless, two ways to practice this. We tin either take one electron pair from each oxygen to course a symmetrical construction or accept both electron pairs from a single oxygen atom to give an asymmetrical structure:
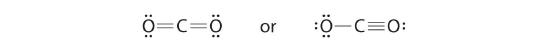
Both Lewis electron structures give all three atoms an octet. How do we decide betwixt these two possibilities? The formal charges for the two Lewis electron structures of CO2 are as follows:
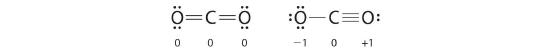
Both Lewis structures take a net formal accuse of zero, merely the structure on the right has a +i charge on the more electronegative atom (O). Thus the symmetrical Lewis structure on the left is predicted to be more than stable, and it is, in fact, the structure observed experimentally. Call up, though, that formal charges do not stand for the actual charges on atoms in a molecule or ion. They are used merely equally a bookkeeping method for predicting the most stable Lewis structure for a compound.
Annotation
The Lewis structure with the set of formal charges closest to zero is usually the almost stable.
Example
The thiocyanate ion (SCN−), which is used in press and as a corrosion inhibitor confronting acidic gases, has at least two possible Lewis electron structures. Draw two possible structures, assign formal charges on all atoms in both, and make up one's mind which is the preferred arrangement of electrons.
Given: chemical species
Asked for: Lewis electron structures, formal charges, and preferred arrangement
Strategy:
A Use the step-by-step procedure to write ii plausible Lewis electron structures for SCN−.
B Calculate the formal charge on each atom using Equation 4.4.1.
C Predict which construction is preferred based on the formal charge on each atom and its electronegativity relative to the other atoms nowadays.
Solution:
A Possible Lewis structures for the SCN− ion are equally follows:
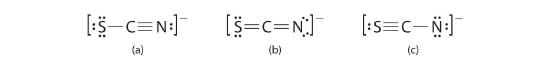
B Nosotros must summate the formal charges on each cantlet to place the more than stable structure. If we begin with carbon, we notice that the carbon atom in each of these structures shares 4 bonding pairs, the number of bonds typical for carbon, so it has a formal charge of zero. Continuing with sulfur, we observe that in (a) the sulfur atom shares one bonding pair and has three solitary pairs and has a total of six valence electrons. The formal charge on the sulfur cantlet is therefore
C Which structure is preferred? Structure (b) is preferred because the negative charge is on the more electronegative cantlet (N), and information technology has lower formal charges on each atom as compared to structure (c): 0, −1 versus +1, −2.
Practise
Salts containing the fulminate ion (CNO−) are used in explosive detonators. Depict three Lewis electron structures for CNO− and utilise formal charges to predict which is more stable. (Note: North is the central cantlet.)
Reply:
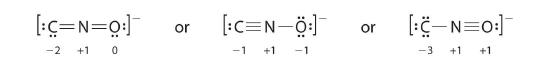
The second construction is predicted to exist more stable.

Cartoon isomers of Lewis Structures
Contributors
- Anonymous
Layne Morsch (Academy of Illinois Springfield)
3 cases can be constructed that do non follow the octet rule, and every bit such, they are known as the exceptions to the octet rule. Post-obit the Octet Rule for Lewis Dot Structures leads to the most accurate depictions of stable molecular and atomic structures and because of this we e'er want to use the octet rule when drawing Lewis Dot Structures. Nonetheless, information technology is difficult to imagine that one rule could exist followed by all molecules. There is always an exception, and in this case, three exceptions. The octet rule is violated in these three scenarios:
- When there are an odd number of valence electrons
- When at that place are too few valence electrons
- When there are as well many valence electrons
Exception 1: Species with Odd Numbers of Electrons
The first exception to the Octet Dominion is when in that location are an odd number of valence electrons. An instance of this would exist Nitrogen (Two) Oxide (NO ,refer to effigy one). Nitrogen has 5 valence electrons while Oxygen has 6. The total would be 11 valence electrons to exist used. The Octet Rule for this molecule is fulfilled in the higher up instance, however that is with 10 valence electrons. The last one does non know where to go. The lone electron is called an unpaired electron. But where should the unpaired electron get? The unpaired electron is commonly placed in the Lewis Dot Structure and so that each element in the structure volition accept the lowest formal accuse possible. The formal charge is the perceived accuse on an individual cantlet in a molecule when atoms do not contribute equal numbers of electrons to the bonds they participate in. The formula to find a formal accuse is:
Formal Charge= [# of valence e- the atom would accept on its own] - [# of lone pair electrons on that atom]
- [# of bonds that atom participates in]
No formal charge at all is the nigh platonic situation. An example of a stable molecule with an odd number of valence electrons would exist nitrogen monoxide. Nitrogen monoxide has 11 valence electrons. If you need more information nigh formal charges, see Lewis Structures. If we were to imagine nitrogen monoxide had x valence electrons we would come upwards with the Lewis Construction (Effigy 8.7.1):
Figure 8.7.one. This is if Nitrogen monoxide has only ten valence electrons, which it does not.
Allow's look at the formal charges of Figure eight.vii.two based on this Lewis structure. Nitrogen normally has five valence electrons. In Effigy eight.7.1, it has two lone pair electrons and information technology participates in ii bonds (a double bail) with oxygen. This results in nitrogen having a formal charge of +1. Oxygen normally has 6 valence electrons. In Figure 8.7.ane, oxygen has four lone pair electrons and it participates in two bonds with nitrogen. Oxygen therefore has a formal charge of 0. The overall molecule here has a formal charge of +1 (+1 for nitrogen, 0 for oxygen. +1 + 0 = +ane). However, if we add the eleventh electron to nitrogen (because we want the molecule to have the everyman total formal charge), it will bring both the nitrogen and the molecule's overall charges to zero, the about platonic formal accuse situation. That is exactly what is done to go the right Lewis structure for nitrogen monoxide (Effigy 8.7.ii):
Effigy 8.7.2. The proper Lewis structure for NO molecule
Gratuitous Radicals
In that location are actually very few stable molecules with odd numbers of electrons that be, since that unpaired electron is willing to react with other unpaired electrons. Near odd electron species are highly reactive, which we phone call Free Radicals. Because of their instability, free radicals bail to atoms in which they can accept an electron from in order to become stable, making them very chemically reactive. Radicals are found as both reactants and products, just generally react to form more than stable molecules as soon every bit they can. In order to emphasize the existence of the unpaired electron, radicals are denoted with a dot in front of their chemic symbol as with
Exception ii: Incomplete Octets
The second exception to the Octet Rule is when in that location are too few valence electrons that results in an incomplete Octet. There are even more than occasions where the octet rule does not give the most right delineation of a molecule or ion. This is also the case with incomplete octets. Species with incomplete octets are pretty rare and by and large are simply found in some glucinium, aluminum, and boron compounds including the boron hydrides. Let'south take a look at one such hydride, BHthree (Borane).
If 1 was to brand a Lewis structure for BH3 post-obit the basic strategies for cartoon Lewis structures, one would probably come up with this structure (Effigy 8.seven.iii):
Figure 8.7.3
The trouble with this structure is that boron has an incomplete octet; it only has half dozen electrons around it. Hydrogen atoms tin can naturally only have just 2 electrons in their outermost shell (their version of an octet), and equally such at that place are no spare electrons to course a double bail with boron. One might surmise that the failure of this structure to grade complete octets must mean that this bond should be ionic instead of covalent. However, boron has an electronegativity that is very similar to hydrogen, meaning at that place is likely very niggling ionic grapheme in the hydrogen to boron bonds, and equally such this Lewis construction, though it does not fulfill the octet rule, is likely the best structure possible for depicting BH3 with Lewis theory. One of the things that may business relationship for BHiii's incomplete octet is that it is commonly a transitory species, formed temporarily in reactions that involve multiple steps.
Let's accept a wait at another incomplete octet state of affairs dealing with boron, BF3 (Boron trifluorine). Like with BH3, the initial drawing of a Lewis construction of BF3 volition class a structure where boron has only six electrons around it (Effigy eight.7.4).
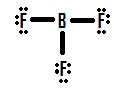
Effigy 8.7.4
If y'all wait Effigy 8.7.4, yous tin come across that the fluorine atoms possess extra lone pairs that they can utilize to make boosted bonds with boron, and you might recollect that all yous have to do is brand one lonely pair into a bond and the construction will be correct. If nosotros add ane double bail between boron and one of the fluorines we go the following Lewis Structure (Figure viii.7.5):
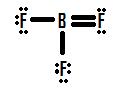
Figure 8.7.5
Each fluorine has eight electrons, and the boron atom has viii equally well! Each atom has a perfect octet, correct? Not then fast. We must examine the formal charges of this structure. The fluorine that shares a double bail with boron has six electrons effectually it (iv from its ii lone pairs of electrons and one each from its ii bonds with boron). This is ane less electron than the number of valence electrons it would have naturally (Grouping Seven elements have seven valence electrons), so it has a formal charge of +ane. The two flourines that share unmarried bonds with boron accept seven electrons around them (half dozen from their three alone pairs and i from their single bonds with boron). This is the same amount equally the number of valence electrons they would have on their ain, so they both take a formal accuse of zero. Finally, boron has four electrons around it (one from each of its four bonds shared with fluorine). This is i more electron than the number of valence electrons that boron would have on its own, and as such boron has a formal charge of -1.
This structure is supported by the fact that the experimentally adamant bail length of the boron to fluorine bonds in BFthree is less than what would be typical for a unmarried bond (see Bond Society and Lengths). However, this structure contradicts 1 of the major rules of formal charges: Negative formal charges are supposed to be found on the more than electronegative cantlet(southward) in a bond, only in the construction depicted in Effigy 8.7.5, a positive formal accuse is constitute on fluorine, which not only is the most electronegative chemical element in the construction, but the most electronegative chemical element in the entire periodic tabular array (
However the large electronegativity difference here, equally opposed to in BHiii, signifies significant polar bonds between boron and fluorine, which means there is a loftier ionic character to this molecule. This suggests the possibility of a semi-ionic structure such as seen in Figure eight.seven.vi:

Figure 8.7.half dozen
None of these iii structures is the "correct" structure in this instance. The near "correct" structure is about probable a resonance of all three structures: the one with the incomplete octet (Figure viii.7.iv), the one with the double bond (Figure 8.7.v), and the i with the ionic bond (Figure viii.vii.6). The most contributing structure is probably the incomplete octet structure (due to Figure 8.vii.five being basically incommunicable and Figure 8.7.6 not matching up with the beliefs and properties of BF3). As you can see even when other possibilities exist, incomplete octets may best portray a molecular structure.
Every bit a side note, it is important to note that BF3 often bonds with a F- ion in order to course BF4 - rather than staying as BFthree. This construction completes boron'south octet and it is more mutual in nature. This exemplifies the fact that incomplete octets are rare, and other configurations are typically more favorable, including bonding with additional ions as in the instance of BF3 .
| Instance eight.7.ane: |
|---|
| Draw the Lewis construction for boron trifluoride (BF3). SOLUTION 1. Add electrons (iii*vii) + three = 24 2. Depict connectivities: 3. Add octets to outer atoms: four. Add together actress electrons (24-24=0) to central atom: five. Does cardinal electron have octet?
6. The primal Boron now has an octet (there would be three resonance Lewis structures) However...
BF3 reacts strongly with compounds which have an unshared pair of electrons which tin exist used to form a bail with the boron: |
Exception 3: Expanded Valence Shells
More mutual than incomplete octets are expanded octets where the central cantlet in a Lewis construction has more eight electrons in its valence trounce. In expanded octets, the primal atom tin can have ten electrons, or even twelve. Molecules with expanded octets involve highly electronegative final atoms, and a nonmetal central atom establish in the third period or below, which those terminal atoms bond to. For case,
| Note |
|---|
| Expanded valence shells are observed only for elements in period 3 (i.eastward. n=3) and across |
The 'octet' rule is based upon available ns and np orbitals for valence electrons (ii electrons in the s orbitals, and 6 in the p orbitals). Beginning with the n=3 principle quantum number, the d orbitals get available (l=2). The orbital diagram for the valence shell of phosphorous is:
Hence, the third catamenia elements occasionally exceed the octet dominion by using their empty d orbitals to accommodate additional electrons. Size is as well an of import consideration:
- The larger the central atom, the larger the number of electrons which can surround it
- Expanded valence shells occur most frequently when the central atom is bonded to small electronegative atoms, such as F, Cl and O.
At that place is currently much scientific exploration and research into the reason why expanded valence shells are found. The top expanse of interest is figuring out where the extra pair(s) of electrons are found. Many chemists think that at that place is not a very large energy departure between the 3p and 3d orbitals, and as such it is plausible for extra electrons to easily make full the 3d orbital when an expanded octet is more favorable than having a consummate octet. This thing is still nether hot debate, however and at that place is even debate every bit to what makes an expanded octet more favorable than a configuration that follows the octet rule.
Ane of the situations where expanded octet structures are treated as more favorable than Lewis structures that follow the octet rule is when the formal charges in the expanded octet structure are smaller than in a construction that adheres to the octet rule, or when there are less formal charges in the expanded octet than in the structure a structure that adheres to the octet rule.
| Case 8.7.2: The |
|---|
| Such is the case for the sulfate ion, And theniv -2. A strict adherence to the octet rule forms the following Lewis structure: Figure eight.seven.12 If we await at the formal charges on this molecule, we tin run across that all of the oxygen atoms have vii electrons around them (six from the three lone pairs and one from the bond with sulfur). This is one more electron than the number of valence electrons and then they would have ordinarily, and equally such each of the oxygens in this structure has a formal charge of -1. Sulfur has four electrons around it in this construction (one from each of its four bonds) which is ii electrons more than the number of valence electrons it would have normally, and as such it carries a formal accuse of +2. If instead nosotros fabricated a construction for the sulfate ion with an expanded octet, it would await like this: Figure viii.7.xiii Looking at the formal charges for this structure, the sulfur ion has six electrons around it (ane from each of its bonds). This is the same amount every bit the number of valence electrons information technology would have naturally. This leaves sulfur with a formal charge of zero. The 2 oxygens that have double bonds to sulfur have half-dozen electrons each around them (four from the two lone pairs and i each from the ii bonds with sulfur). This is the same corporeality of electrons as the number of valence electrons that oxygen atoms take on their own, and every bit such both of these oxygen atoms have a formal accuse of nix. The two oxygens with the single bonds to sulfur have 7 electrons around them in this structure (six from the three lone pairs and one from the bond to sulfur). That is 1 electron more than than the number of valence electrons that oxygen would have on its own, and as such those 2 oxygens carry a formal charge of -one. Remember that with formal charges, the goal is to keep the formal charges (or the difference betwixt the formal charges of each atom) equally pocket-size every bit possible. The number of and values of the formal charges on this structure (-i and 0 (divergence of 1) in Figure 8.7.12, as opposed to +2 and -i (difference of iii) in Figure 8.7.12) is significantly lower than on the construction that follows the octet rule, and as such an expanded octet is plausible, and even preferred to a normal octet, in this case. |
| Case 8.seven.3: The |
|---|
| Draw the Lewis structure for SOLUTION 1. Count upward the valence electrons: 7+(4*vii)+1 = 36 electrons ii. Draw the connectivities: 3. Add octet of electrons to outer atoms: 4. Add actress electrons (36-32=4) to fundamental atom: 5. The ICl4 - ion thus has 12 valence electrons around the central Iodine (in the 5d orbitals) |
Expanded Lewis structures are besides plausible depictions of molecules when experimentally adamant bail lengths suggest partial double bond characters even when single bonds would already fully fill up the octet of the central atom. Despite the cases for expanded octets, as mentioned for incomplete octets, it is important to keep in mind that, in general, the octet dominion applies.
Source: https://chem.libretexts.org/Courses/Winona_State_University/Klein_and_Straumanis_Guided/1%3A_A_Review_of_General_Chemistry_-_Electrons%2C_Bonds%2C_and_Molecular_Properties/1%3A2_Electron-Dot_Model_of__Bonding%3A_Lewis__Structures_and_Formal_Charges

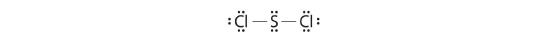


0 Response to "3d drawing of hydrogen fulminate"
Post a Comment